Water is not just for drinking.
It is something we take for granted. It covers over 70% of the Earth’s surface. Life has evolved so it can’t go on without it. We know it as H2Oand yet once upon a time it was just water.



In the 1700’s, water was considered elemental. Part of the whole ‘Fire, Water, Earth & Air” thing espoused by the ancients (Greeks mostly and the film ‘The 5th Element’). That is- a thing in its own entity. Not made up of the gasses Oxygen and Hydrogen, but a thing – like a lump of iron, pure- water. Not molecules and certainly not chemicals. And yes. Water is a chemical. Get over it.
2H2 + O2 → 2H2O
Discoveries in chemistry enabled us to see the world as never before. Henry Cavendish (1731-1810) who discovered that water was made up of smaller parts and wasn’t elemental in its own right, named it first as ‘dephlogisticated air‘. It’s a bit of a mouthful and you can see why that name didn’t stick.
There are so many things about water that we take for granted- It evaporates easily, makes steam, freezes and dissolves things, for starters.
If you think about the freezing thing. Yes, you may know it expands upon freeezing (cracked pipes in winter and water bottles in the freezer filled too high), and you know for sure that it floats-icebergs and polar bears on icebergs, but out of interest can you list how many other things in their solid form float when they freeze?

Most things sink when they freeze. Think about that.
If we didn’t have ice floating what would happen to the world? Well- if ice sank to the bottom of the sea, apparently the whole of the body of water would freeze solid- and most aquatic life as we know it wouldn’t survive. Then all the life that depends on that aquatic life would suffer and so on….

It also dissolves things easily too- coffee granules, sugar, salt and molecules in your blood. And when you drop your plugged-in-iphone into the bathwater it’s the dissolved salts and other things that help electrocute you.
Aside from it’s behaviour it is mesmerising, beautiful and blue.
For those of you that have seen the sea, it is blue- but in your water bottles, or even buckets of water from the beach- it looks clear. So why is the sea blue?
It’s a matter of volume really and the blue colour is soooooo dilute (literally), small volumes of water won’t have enough colour in them to be detected by the naked eye. If you collect enough water together though, it’ll look blue and it’s blue because it reflects blue light only.
So where does the red, yellow and orange light in the spectrum go when white light is hitting the water?
Well, it gets absorbed (with added impurities for good measure more light is scattered and the clarity of the blueness is diminished somewhat). Below is a nice guide to how we see colour from direct white light.

Even more than floating and its colour though, is this wonderful ability to form water droplets and bubbles- and this is because of charges on the tiny molecules themselves. A positive and a negative charge- (negative charges carry electricity to give you an inkling).
So now to explain….
 Chemistry: Oxygen is big and fat and has charge. Hydrogen ions are tiny and needy and attacted to elements like oxygen that are bigger and can give them what they want. They want charge (electrons).
Chemistry: Oxygen is big and fat and has charge. Hydrogen ions are tiny and needy and attacted to elements like oxygen that are bigger and can give them what they want. They want charge (electrons).
So they meet up. This co-dependent relationship. Ideally, they would ‘Share’ electrons, Oxygen needs what Hydrogen has to offer and Hydrogen needs what Oxygen has to make it complete.
 So they hook up, but the bigger, fatter Oxygen has lots more negative charge (electrons) than the hydrogen, so the electrons that they ‘share’, stay close to the oxygen, and poor old hydrogen in its co-dependent needy relationship is bereft of what it wanted most- yes it’s still complete, but actually has lost it’s negativity and become a positively charged ion.
So they hook up, but the bigger, fatter Oxygen has lots more negative charge (electrons) than the hydrogen, so the electrons that they ‘share’, stay close to the oxygen, and poor old hydrogen in its co-dependent needy relationship is bereft of what it wanted most- yes it’s still complete, but actually has lost it’s negativity and become a positively charged ion.
So now our H2O has a positive side and a negative side. The size of the Oxygen, overwhelming in its fatness and negativity, causes the hydrogen to exist at an angle the furthest away from Oxygen that it can possibly be. So it exists as a V shape or Mickey Mouse ears. A bit like when you see Leia backing up from Jabba the Hutt.
This polarity- negative one side and positive the other, gives water those properties we take so much for granted and I don’t mean that it just dissolves candyfloss.
Now, opposites attract, and so the positive Hydrogen is attracted to other Oxygens (negative) within the water- and this force of attraction is called a Hydrogen bond. Likewise, the Oxygen wants to play away too- so is attracted to other Hydrogens. Water molecules bonds loosely to other water molecules- the positively charged parts attracted to the negative- you’ll have seen this in magnetism. Sort of.

When they’re in the liquid state, those little molecules are sliding all over each other, and these attractive forces mean they’ll not evaporate unless you boil them.
What this means is that with these Hydrogen bonds within the water it takes a bit more effort and energy to get the water to boil and turn into a vapour or gas. Now the important bit: If those qualities of H-bond attraction didn’t exist, water wouldn’t be liquid at room temperatures it would be a gas and we’d be dead as there would be no liquid water on the planet, not to mention our veins would be full of air- oh and we wouldn’t exis……
Now because water is fluid, you don’t appreciate much of these shenannigans until things get extreme- i.e very very cold or when water meets air.
When it freezes the water molecules attracted to each other align themselves.
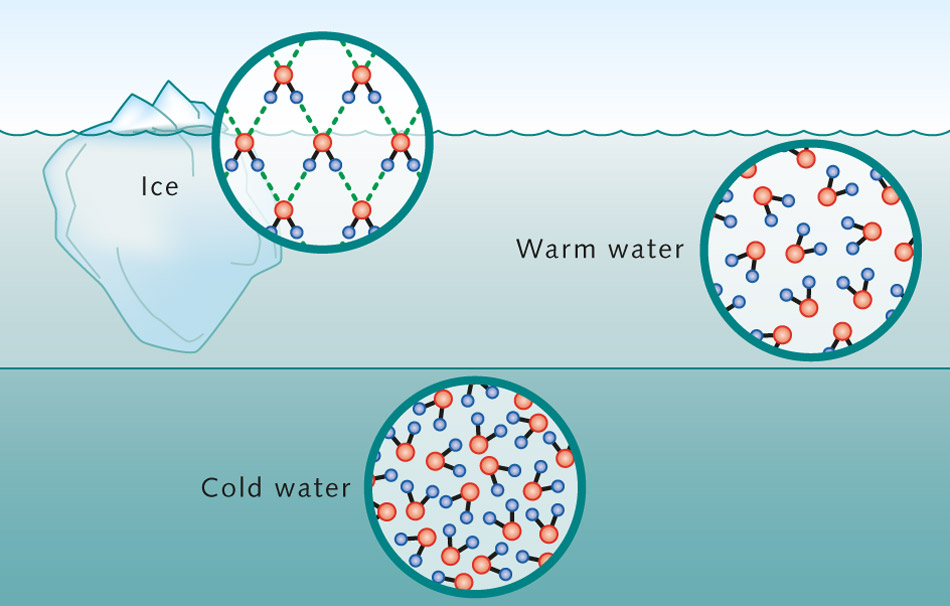
Hydrogen bonds keep the molecules at an appropriate distance for conformity- a bit like when soldiers have to line up an arms length from each other. And these alignments are strong- don’t forget the burst frozen pipes. In maintaining this precise distance, water increases by about 9% in volume, becomes less dense than liquid water and so can float.

When it comes to contact with air, what does water do?
Droplets.
Droplets are also due to the charge that water carries .We refer to this as surface tension and you may have seen it if you ever look at a water boatman or a mosquito on water. They are not heavy enough to penetrate the surface.
How does it create tension at the surface? Well. You know by now water is charged. Usually, these forces pull any one water molecule that is surrounded by other water molecules in all directions. At the edge though, there is nothing. No charge. Nowt but the air. So the charges that exist face inwards making these water molecules at the surface more strongly attracted to each other.


These characteristics of water also contribute to other behaviours like capillary action- and also the refraction of light and crystallisation of water- but snowflakes and crystals are for another time…
So the next time you’re drinking your aqua vita think of how amazing this molecule is.