6 simple and effective exam revision secrets.
Exams………..HUH……….YEAH…………………..what are they good for?

I wish I could say absolutely nothing.
At the most basic level they give you a qualification that will help you get a job
– and that I find is exactly where my problem with exams exists. You end up in the situation where people can do very well in exams, can perform, can self promote, but ultimately are as thick as stumps and quite useless. (We ALL know folks like this). I digress.
Currently, exams are the best and easiest measure to see how far or how well people are doing.
To be honest, I wasn’t good at exams until I left school and went to university. It was my inability to conform I think. At university I was free and stopped trying to understand what the teacher wanted, and figured what was required from the syllabus independently. I began to excel. On my terms, exams became my own measure- I did my best, I tried my best I should say, and if that wasn’t good enough, then clearly I shouldn’t be there.
My best is all I can do , if its not good enough- then so be it.
It took the pressure off during exams and is quite a fatalistic approach- but it worked for me.
I say the same to the pharmacy students I teach- If you don’t know this stuff- you shouldn’t be a pharmacists- ultimately you’ll end up killing people.
Now when you’re; 16,17,18,19,20,40……whatever age – although it’s not life and death on those terms, exams may feel like it because you have no control.
So what changed between school and university?
It was a mindset: To succeed at exams you must take back CONTROL and carry them out on your terms.
So, here are my tips on how you should do that based on my experiences as a student and as an educator…..
1. Figure out what you are doing these exams for.
Sometimes it’s; parents, pressure, expectation and you’re doing these exams with no clue as to why. Will you really need Chemistry? Maths? Drama?
The straight answer is: Yes. (soz)
Not in a direct way, like; I’d like to be able to use chemistry to coat all metal in my house in gold, or colour everything green, but chemists have skills in deductive reasoning, step by step equations, thinking outside the box.
Maths- OK, I’ve never applied the equations under a curve in all my life since I was 18, but finding patterns, solving puzzles- that’s what maths gives you.
Drama: Will I ever be on stage? Nah. However, I could learn confidence, clarity of speech-(great for oratory skills).
These skills help you, you get the picture.

When I look at university admissions, it’s not so much the subjects you do , but the fact that you’ve done them at all. Gone through that pain, willpower and discipline to get to a point that you can apply for university or for a job. We’ve all been through it. We feel your pain. We respect that.
It’s the same as going to the gym- why do people do it? To get fit, look fit, get healthier- numerous reasons- but because you can see an effect it’s worth the effort, all those mindblowingly boring excercises. Bleurgh. This educational process is the same- sometimes mindblowingly boring- like having your soul sucked right out of you, and you can’t immediately see the effects.
So what are you doing this for?
Answer: To give yourself a different skill set that will give you options, put you on a journey. It’s painful, but you are gaining discipline at a different level. Look after yourself, take pride in what you have acheived and develop yourself even if you can’t immediately see any value in it.
When people say ‘it’s hard work’- its this pain and discipline that they’re referring to. People always respect hard work.
Once you’ve mastered discipline, you are then testing yourself to see how well you have done.

Excercise: What skills will your most hated subject give you?
2. Is it too overwhelming?
Well, duh. Yes.
Be brave.
Take the first step. The first step is one more step than previously, so you are progressing. Slowly, but if you continue you will be further ahead than you were before you started.
If you never start- you will never start and a year from now you will still be in the same place.
Apart some sounding all wise, the truth is that once people start, they feel better and the anxiety diminishes.

If you are about to start revision and know NOTHING – you are learning for the first time and not really revising. Revising and ‘new’ learning are two different things in my book.
Learning everything from scratch takes time, it will take time to grasp concepts- so give yourself extra time before you hit revision. You’ll need to spend time watching youtube videos or even a book or two so you can grasp what’s what.
It is useful during whatever course you take to make sure you understand stuff. If you don’t-DON’T WAIT TIL REVISION TO SORT IT OUT- this takes too long. But if you have left it late, don’t lose hope. Give yourself extra time in your revision/ learning timetable – (probably give up on hopes of lie ins)- or simply put- just start.
Whatever. Start. Go on. Start. Now.
3. Set your own pace for revision
We want everything immediately, it’s in our nature, but if you chip away at something, then untimately you make change happen.

Figure out your own pace of learning- then figure out your destination (what must you have learned by the end of revision) then make a plan of how long you estimate it will take to get there; for example:
You have a syllabus. USE IT. If you can manage to learn only 1 topic of a particular subject a week, and you know you have at least 20 topics within that subject to be examined on, ask yourself the following:
Do you understand the concepts in the syllabus?- If yes: you are revising-If no:you are learning for the first time.
If you are revising, how many times/how often do you have to repeat it before it will go in? Usually for me I had to go over the same thing on at least 3 different occasions during revision- I was a slow reviser and knew my limitations, plus I literally had my room, house and toilet door covered in Post-it notes of things to remember or pictures.
From this, figure out how much time you need to revise- including rest stops, days off and at the end, going over exam papers to be properly polished for your exam.
Once you have figured all of that out- work out how much time (realisitcally) you will commit to getting to that level. Again, do include in this the time to rest and relax- burnt out students are good for no-one.
4. NEVER, EVER, EVER compare yourself to others (unless by some twist of fate and intelligence you are top- in which case well done- and why are you reading this?)
Ultimately you are your own gold standard.
If you are revising and you have leapt up from 30% in an exam to 40%- that, my friend, is a significant improvement. Keep going.

5. Keep going.
Again, true as in life, I believe sometimes people are only where they are because they’re the last man standing. Pure tenacity and staying power have kept them there. Don’t give up and you’ll get there. You can surprise yourself when setting yourself these tenacious challenges as to how far you can actually go. You will find that each time you push yourself, you will have acheived much more than you previously had ever thought possible.
6. Practise.
So you’ve revsied as much as you are able- but is it good enough?
Well, your best is all you can do, literally, but sometimes applying a bit of savvy to the process helps a lot.
- Practice exam questions to give you a number of perspectives on what you had thought you had learnt.
- Learn to articulate what you have learnt.
- Teach others.
- Write it all down, write essays.
All of these processes help.
Finally- some of you are night owls, and burn the midnight oil. This is bad news if you have a 9am exam. Try and revise during the periods that your exams will be set, otherwise you will just be knackered and not your physical best, especially the week before your exam.
Summary:
Most of these lessons apply to life. Learn them now and it makes certain paths you will travel easier. You will learn them sooner or later though, just make it so that you can apply them before you grow too old to use them.
If you do have any questions to help you with revision techniques please post them in the comments section and I’ll be more than happy to help you work through them.
Good Luck!






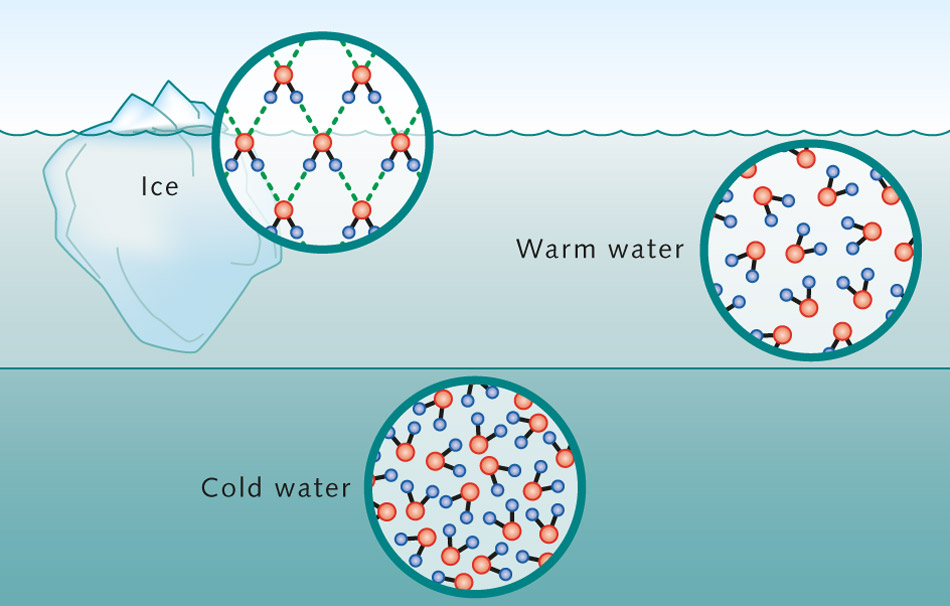
 Chemistry: Oxygen is big and fat and has charge. Hydrogen ions are tiny and needy and attacted to elements like oxygen that are bigger and can give them what they want. They want charge (electrons).
Chemistry: Oxygen is big and fat and has charge. Hydrogen ions are tiny and needy and attacted to elements like oxygen that are bigger and can give them what they want. They want charge (electrons). So they hook up, but the bigger, fatter Oxygen has lots more negative charge (electrons) than the hydrogen, so the electrons that they ‘share’, stay close to the oxygen, and poor old hydrogen in its co-dependent needy relationship is bereft of what it wanted most- yes it’s still complete, but actually has lost it’s negativity and become a positively charged ion.
So they hook up, but the bigger, fatter Oxygen has lots more negative charge (electrons) than the hydrogen, so the electrons that they ‘share’, stay close to the oxygen, and poor old hydrogen in its co-dependent needy relationship is bereft of what it wanted most- yes it’s still complete, but actually has lost it’s negativity and become a positively charged ion.